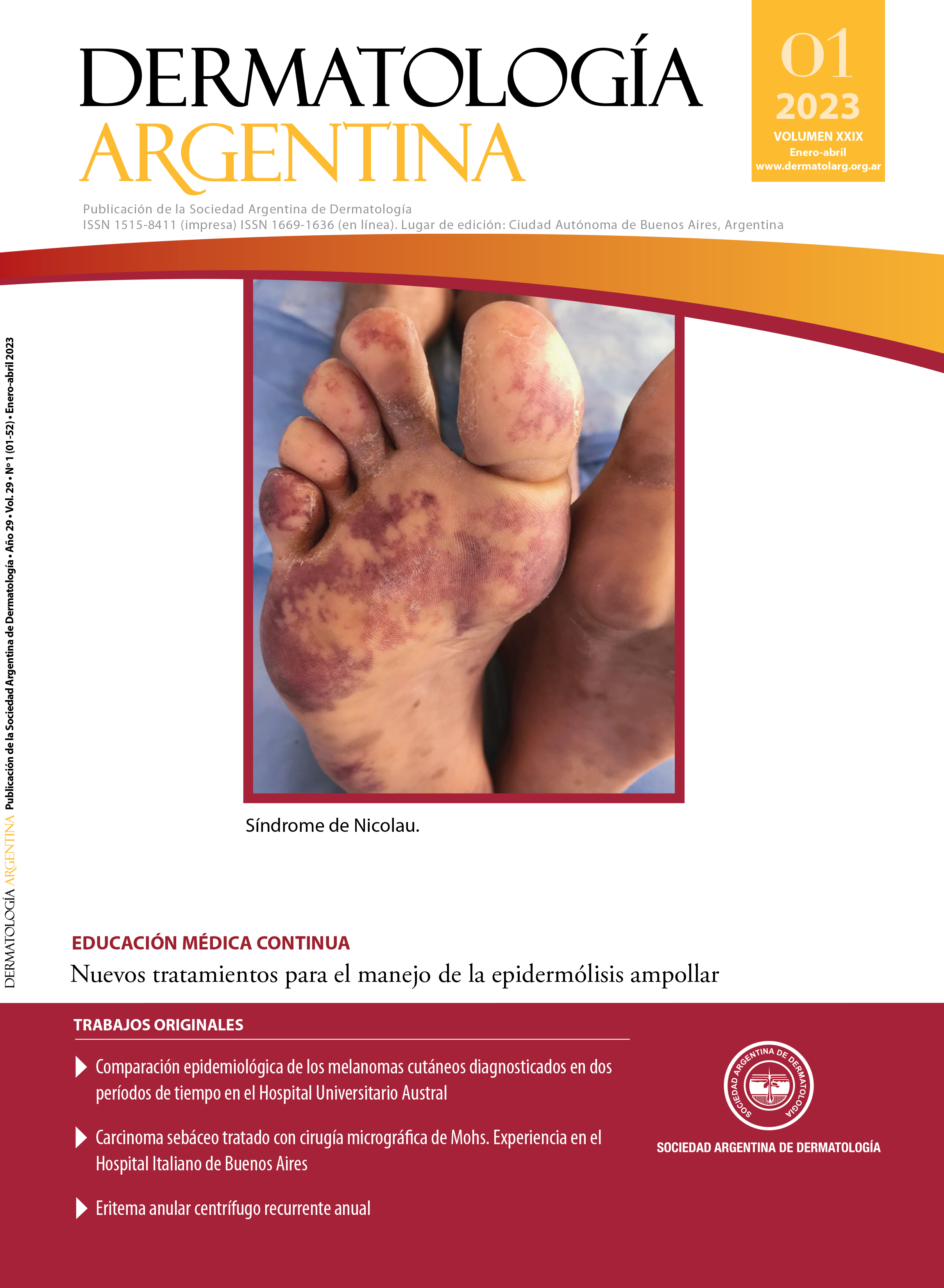
New treatments for epidermolysis bullosa management
DOI:
https://doi.org/10.47196/da.v29i1.2312Keywords:
epidermolysis bullosa, treatment, gene therapyAbstract
Epidermolysis bullosa is a low prevalence genodermatosis family. Characterized for high mechanical fragility of the involved tissues leading to mucocutaneous blistering, erosions and ulceration that are difficult to
manage.
The wide phenotypic spectrum of the disease goes from a mild compromise with cutaneous involvement exclusively to the most severe phenotypes where not only skin but mucous membranes can be affected, becoming a disabling and even fatal multisystemic disease.
Besides this disease is considered a paradigm in the biology of the skin since its study allowed fundamental advances in the understanding of the functioning of the basement membrane, nevertheless currently, the
management of the disease continues to be palliative since there is still no approved treatment available directed towards its pathophysiology.
However, in the past years, advances in gene therapy research have shown important approaches towards future treatments that may change these patients' lives.
The objective of this work is to review the most recent advances in EB treatments, emphasizing the latest discoveries.
References
I. Bardhan A, Bruckner-Tuderman L, Chapple ILC, Fine JD, et ál. Epidermolysis bullosa. Nat Rev Dis Primers. 2020;6:78-105.
II. Siañez-González C, Pezoa-Jares R, Salas-Alanís JC. Epidermólisis ampollosa congénita: revisión del tema. Actas Dermosifiliogr. 2009;100:842-856.
III. Uitto J, Pulkkinen L. Epidermolysis bullosa in Mexico. Int J Dermatol. 2000;39:433-435.
IV. Abahussein AA, al-Zayir AA, Mostafa WZ, Okoro AN. Epidermolysis bullosa in the Eastern Province of Saudi Arabia. Int J Dermatol. 1993;32:579-581.
V. Shinkuma S, Natsuga K, Nishie W, Shimizu H. Epidermolysis bullosa in Japan. Dermatol Clin. 2010;28:431-432.
VI. Fine JD. Epidemiology of inherited epidermolysis bullosa based on incidence and prevalence estimates from the National Epidermolysis Bullosa Registry. JAMA Dermatol. 2016;152:1231-1238.
VII. Monteavaro ML, Tosetto SJ, Schuler-Faccini L, Kiszewski AE. Inherited epidermolysis bullosa: update on the clinical and genetic aspects. An Bras Dermatol. 2020;95:551-569.
VIII. Bageta ML, Cella E, Martínez MF, Ledo GN, et ál. Características clínicas y epidemiológicas de los pacientes con epidermólisis ampollar congénita confirmada por estudio molecular. Estudio retrospectivo en un hospital pediátrico. Dermatol Argent. 2019;25:152-160.
IX. Has C, Bauer JW, Bodemer C, Bolling MC, et ál. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
X. Welponer T, Prodinger C, Pinon-Hofbauer J, Hintersteininger A, et ál. Clinical Perspectives of gene-targeted therapies for epidermolysis bullosa. Dermatol Ther. 2021;11:1175-1197.
XI. Hou PC, Wang HT, Abhee S, Tu WT, et ál. Investigational treatments for epidermolysis bullosa. Am J Clin Dermatol. 2021;22:801-817.
XII. Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, et ál. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397-1402.
XIII. Hirsch T, Rothoeft T, Teig N, Bauer JW, et ál. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327-332.
XIV. Eichstadt S, Barriga M, Ponakala A, Teng C, et ál. Phase 1/2a clinical trial of gene-corrected autologous cell therapy for recessive dystrophic epidermolysis bullosa [en linea]. JCI Insight 2019;4:e130554. Disponible en: <https://insight.jci.org/articles/view/130554> [Consultado julio 2022].
XV. Lwin SM, Syed F, Di WL, Kadiyirire T, et ál. Safety and early efficacy outcomes for lentiviral fibroblast gene therapy in recessive dystrophic epidermolysis bullosa [en línea] JCI Insight 2019;4:e126243. Disponible en: <https://insight.jci.org/articles/view/126243> [Consultado julio 2022].
XVI. Gurevich I, Agarwal P, Zhang P, Dolorito JA, et ál. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. 2022;28:780-788.
XVII. Hainzl S, Peking P, Kocher T, Murauer EM, et ál. COL7A1 Editing via CRISPR/Cas9 in Recessive Dystrophic Epidermolysis Bullosa. Mol Ther. 2017;25:2573-2584.
XVIII. Anzalone AV, Randolph PB, Davis JR, Sousa AA, et ál. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149-157.
XIX. Umegaki-Arao N, Pasmooij AMG, Itoh M, Cerise JE, et ál. Induced pluripotent stem cells from human revertant keratinocytes for the treatment of epidermolysis bullosa. Sci Transl Med. 2014;6:1-10.
XX. Matsumura W, Fujita Y, Shinkuma S, Suzuki S, et ál. Cultured epidermal autografts from clinically revertant skin as a potential wound treatment for recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2019;139:2115-2124.
XXI. Gostyński A, Pasmooij AMG, Jonkman MF. Successful therapeutic transplantation of revertant skin in epidermolysis bullosa. J Am Acad Dermatol. 2014;70:98-101.
XXII. Goto M, Sawamura D, Nishie W, Sakai K, et ál. Targeted skipping of a single exon harboring a 4 premature termination codon mutation: implications and potential for gene correction therapy for selective dystrophic epidermolysis bullosa patients. J Invest Dermatol. 2006;126:2614-2620.
XXIII. Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, et ál. Bone Marrow Transplantation for Recessive Dystrophic Epidermolysis Bullosa. N Engl J Med. 2010;363:629-639.
XXIV. Conget P, Rodriguez F, Kramer S, Allers C, et ál. Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy. 2010;429-431.
XXV. Woodley DT, Keene DR, Atha T, Huang Y, et ál. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat Med. 2004;10:693-695.
XXVI. Remington J, Wang X, Hou Y, Zhou H, et ál. Injection of recombinant human type VII collagen corrects the disease phenotype in a murine model of dystrophic epidermolysis bullosa. Mol Ther. 2009;17:26-33.
XXVII. Cogan J, Weinstein J, Wang X, Hou Y, et ál. Aminoglycosides restore full-length type VII collagen by overcoming premature termination codons: therapeutic implications for dystrophic epidermolysis bullosa. Mol Ther. 2014;22:1741-1752.
XXVIII. Woodley DT, Cogan J, Hou Y, Lyu C, et ál. Gentamicin induces functional type VII collagen in recessive dystrophic epidermolysis bullosa patients. J Clin Invest. 2017;127:3028-3038.
XXIX. Li Y, Shen J, Liang J, Zheng L, et ál. Gentamicin induces COL17A1 nonsense mutation readthrough in junctional epidermolysis bullosa. [en línea] J Dermatol. 2020;47:e82-83. [Consultado julio 2022].
Downloads
Published
Issue
Section
License
Copyright (c) 2023 on behalf of the authors. Reproduction rights: Argentine Society of Dermatology

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
El/los autor/es tranfieren todos los derechos de autor del manuscrito arriba mencionado a Dermatología Argentina en el caso de que el trabajo sea publicado. El/los autor/es declaran que el artículo es original, que no infringe ningún derecho de propiedad intelectual u otros derechos de terceros, que no se encuentra bajo consideración de otra revista y que no ha sido previamente publicado.
Le solicitamos haga click aquí para imprimir, firmar y enviar por correo postal la transferencia de los derechos de autor