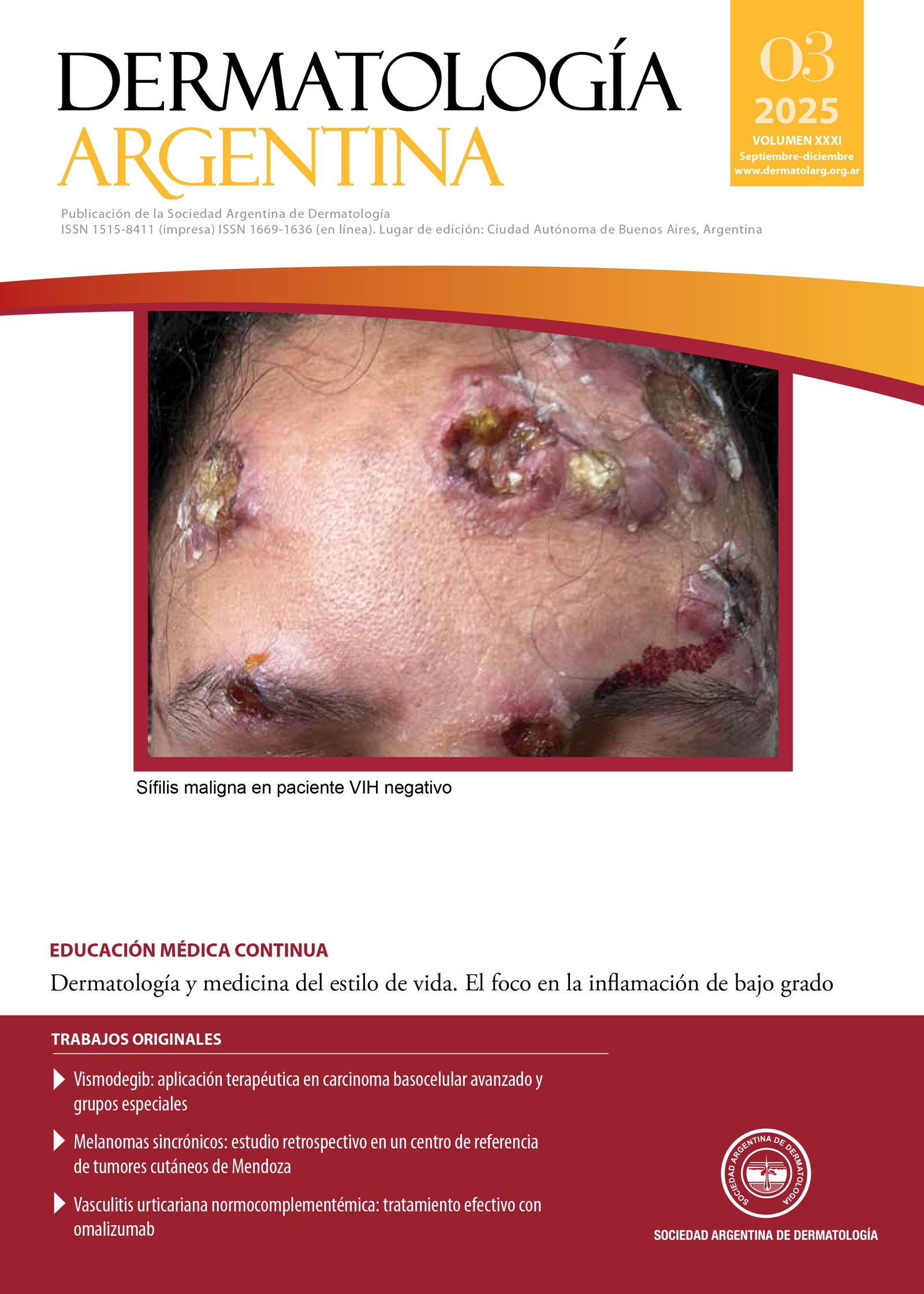
Vismodegib: therapeutic application in advanced basal cell carcinoma and special groups. Experience at the Prof. A. Posadas Hospital
DOI:
https://doi.org/10.47196/da.v31i3.2957Keywords:
basal cell carcinoma, Hedgehog, vismodegibAbstract
Introduction: basal cell carcinoma (BCC) accounts for 75% of non-melanoma skin cancers. Vismodegib is a Hedgehog signaling pathway inhibitor approved by the FDA (Food and Drug Administration) in 2012 for the treatment of locally advanced BCC (LA-BCC). Regarding the response to treatment, a partial or complete decrease in tumor mass is evident. Adverse effects are generally mild. The most frequent are: cramps, alopecia, dysgeusia, diarrhea, fatigue, asthenia, among others.
Objectives: demonstrate the response to treatment with vismodegib in patients with BCC-LA, metastatic and special groups, evaluated from 2/1/2018 to 29/12/202, at Posadas Hospital. Describe the variables: sex, age, number of lesions, location, size, histological subtype, time of evolution, primary/recurrence, treatment duration, response to treatment, adverse effects, medical coverage, discontinuation due to toxicity and discontinuation due to lack of access to medication.
Materials and methods: descriptive and retrospective study. A search was conducted of photographic archives and medical records of patients with BCC-LA who received vismodegib.
Results: 31 patients with BCC-LA, including 2 metastatic, 1 Gorlin syndrome and 1 chronic endemic hydroarsenicism (CEHA). Complete response was observed in 11 (35.5%) patients and partial response in 13 (41.9%). Regarding toxicity, 18 (58%) patients presented adverse effects and 7 (22.6%) suspended treatment due to intolerance.
Conclusions: Hedgehog pathway inhibitors are novel treatments for LA-CBC. They represent a great therapeutic alternative, thus avoiding mutilating surgeries. In our population, response rates like those described in the literature were obtained. Although adverse effects are common, the drug has a mild toxicity profile.
References
I. Pulido-Prieto L, Esguerra-Cantillo JA, Toquica-Díaz NA, Ospina-Delgado MA. Terapia multimodal con vismodegib y radioterapia en el tratamiento del carcinoma basocelular localmente avanzado: reporte de cuatro casos. Actas Dermosifiliogr. 2023;114:264-267.
II. Pulido L, Ospina M, Jaime AO, Carreño J. Terapia intermitente con vismodegib para el CBC avanzado: experiencia en un centro de referencia oncológico en Colombia. Rev Colomb Cancerol. 2022;26:403-411.
III. Loizate-Sarrionandia I, Hernández-González R, Suárez-Hernández J, Fernández de Misa Cabrera R. Combinación de terapias en el carcinoma basocelular localmente avanzado. De la teoría a la práctica clínica. Actas Dermosifiliogr. 2024;115:508- 510.
IV. Lear JT, Corner C, Dziewulski P, Fife K, et ál. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111: 1476-1481.
V. Khoo ABS, Ali FR, Lear JT. Defining locally advanced basal cell carcinoma and integrating smoothened inhibitors into clinical practice. Curr Opin Oncol. 2016; 28: 180-184.
VI. Schmults CD, Blitzblau R, Aasi SZ, Alam M, et ál. Basal cell skin cancer. J Natl Compr Canc Netw. 2023;21:1181-203.
VII. LoRusso PM, Rudin CM, Reddy JC, Tibes R, et ál. Phase I trial of Hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502- 2511
VIII. Kurnia-Wijaya J, Wahab S, Nurdin A, Iran Anwae A. Vismodegib y sonidegib en el carcinoma de células basales localmente avanzado y metastásico: actualización acerca de los inhibidores de la vía de Hedgehog. Actas Dermosifiliogr. 2022;113:443- 450.
IX. Ruiz-Salas V, Alegre M, López-Ferrer A, Garcés JR. Vismodegib: revisión. Actas Dermosifiliogr. 2014;105:744-751.
X. Axelson M, Liu K, Jiang X, He K, et ál. U.S. Food and Drug Administrarion approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
XI. Passarelli A, Galdo G, Aieta M, Fabrizio T, et ál. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596.
XII. Campastri A, Bordón MP, Cilio AM, Foenquinos R, et ál. Experiencia del uso de vismodegib para el tratamiento del carcinoma basocelular. Dermatol Argent. 2022;28: 119-124.
XIII. Bernia E, Llombart B, Serra- Guillén C, Bancalari B. Experiencia con vismodegib en carcinoma basocelular avanzado en un centro oncológico. Actas Dermosifiliogr. 2018; 109:813-820
XIV. Sekulic A, Migden MR, Basset- Seguin N, Garbe C, et ál. Long- term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
XV. Basset-Seguin N, Hauschild A, Grob JJ, Kunstfeld R, et ál. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729-736.
XVI. Tang JY, Mackay Wiggan JM, Aszterbaum M, Yauch RL, et ál. Inhibiting the Hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180-2188.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 on behalf of the authors. Reproduction rights: Argentine Society of Dermatology

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
El/los autor/es tranfieren todos los derechos de autor del manuscrito arriba mencionado a Dermatología Argentina en el caso de que el trabajo sea publicado. El/los autor/es declaran que el artículo es original, que no infringe ningún derecho de propiedad intelectual u otros derechos de terceros, que no se encuentra bajo consideración de otra revista y que no ha sido previamente publicado.
Le solicitamos haga click aquí para imprimir, firmar y enviar por correo postal la transferencia de los derechos de autor